Customizing brine
Snow-removal costs can represent a significant portion of the road-maintenance budget for cities and states.
Liquid brine solution is spray-applied in advance of a snow weather event.
In addition, deicing chemicals increase chloride levels in shallow groundwater and surface water, which can lead to significant and expensive water-quality issues. Benjamin Franklin once said, “An ounce of prevention is worth a pound of cure.” Cities and states can reduce their winter-maintenance costs and be stewards of the environment while ensuring public safety by optimizing deicing chemical usage and snowplow routing. This article highlights how the city of Lincoln, Neb., has dramatically decreased deicing chemical use while maintaining safe roadways in the city.
A brine specimen made during the crystallization study. Sixty different brine recipes across a range of temperatures were made.
Bulk calcium chloride prills are converted into a brine with a precise specific gravity.
Before & after
Before 2014 the city of Lincoln only applied anti-icing brine solution to bridge decks and major intersections before winter weather events were forecasted. Much of the brine used was a proprietary MgCl2-based solution purchased from a supplier. In 2014, the city implemented a plan to transition its primary snow- and ice-melting method from spreadable granular material to sprayable liquid brine. Using brine would allow the city to optimize brine blends for each winter event, maximizing the melting capability while minimizing cost and chloride loadings. Salt dissolved in water to make sprayable brine allows the city to be more precise during the application process, using less salt to treat 1,200 lane-miles of emergency snow, school and commuter bus routes and highways. The brine is applied before an anticipated snow fall event as an anti-icing agent. During and after the weather event, brine is used to pre-wet granular rock salt (NaCl) when conducting spreading operations to help prevent the salt from bouncing off to the road surface and scattering, leading to ineffective use. In the future, Lincoln will use the brine alone as a deicing agent as well. Using less salt can translate into savings while minimizing corrosive effects and environmental impact.
The city equipped 10 tanker trucks to carry and apply the brine using pumps and spray nozzles. In addition, trucks that spread granular material were equipped with saddle tanks to spray the granular material as it was being spread. A mixing and storage facility was constructed to prepare and store the brine solution, allowing salts used to make the brine to be purchased and stored in bulk granular form. The brine solution was composed of water, NaCl and CaCl2 depending on the expected conditions during the winter-weather event. Additional food processing byproducts such as cheese, pickle, potato or beet juice were often incorporated into mixtures depending on the region. Lincoln used beet juice in the deicing solution to increase the viscosity and help reduce the bounce and scatter of material during the application process and improve the residual effects of deicers, allowing fewer reapplications.
The learning curve
The change from purchasing brine to making brine in-house did not come without a learning curve. The first few batches of brine that were mixed consisted of concentrated NaCl made at room temperature. When the tanker trucks were loaded, the temperature of the brine dropped significantly due to the winter weather and some of the NaCl crystallized, coming out of solution. The brine containing the dissolved salt had become a slurry with solid salt crystals suspended in the brine. These solid salt crystals clogged spray nozzles in the tanker trucks. Since the brine was initially made indoors, it was relatively easy to dissolve the NaCl to make a high weight-percent solution. But as temperatures decreased, the solubility decreased and the salt could no longer stay in solution, causing crystallization. This problem was quickly remedied by mixing a lower weight-percent NaCl solution that would not crystallize at lower temperatures. The city prepared the brine to be at a certain specific gravity, ensuring the solution contained the desired weight percent of NaCl.
Brines containing only NaCl are effective down to certain temperatures. For example, when the temperature dips below 14°F, the rate at which ice melts declines, and the NaCl-based brine becomes ineffective at melting ice altogether at temperatures below -6°F. To melt ice at lower temperatures and at greater rates, MgCl2 and CaCl2 can be added to the brine but not without consequence. MgCl2 and CaCl2 are significantly more expensive than NaCl, contain higher weight-percent chloride content, and have a higher impact on the durability of concrete. In an effort to make brine that would be effective at lower temperatures, additional stock solutions of MgCl2 and CaCl2 were evaluated. Although the individual stock solutions of NaCl, MgCl2, and CaCl2 would not crystallize at lower temperatures by themselves, mixing a portion of the NaCl brine with MgCl2 brine or CaCl2 brine resulted in crystallization. At this point, a decision was made to investigate deicing brines with the goal to reduce deicing material use and improve efficiency. The city made an arrangement with the University of Nebraska-Lincoln to access equipment in order to test various brine mixtures for crystallization and for melting effectiveness at various temperatures ranging from -15°F to 32°F.
The study
The goal of the study was to produce eight different brine blends that could be used depending on the forecasted temperature during the winter-weather event. The first blend would contain only NaCl and be effective at temperatures above 14°F. Each of the remaining seven blends would contain CaCl2 at increasing concentrations as expected temperatures dropped. A freezer system with an adjustable temperature set-point in the William A. Scheller Chemical Engineering Biochemical Research Laboratory was used to cool brine samples. Each sample contained 10% beet juice, 0-60% stock CaCl2 brine, and the balance of NaCl brine on a volume basis. The specific gravity of the NaCl brine for each blend was lowered until salt crystallization was no longer observed at the target temperature range. Once a formulation was created to avoid salt crystallization, the effectiveness of the brine at melting ice was tested. Ice cubes were submerged in the brine for a set amount of time and then weighed to determine the rate at which the ice melted.
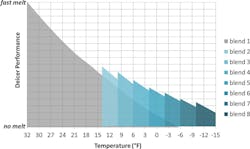
Figure 1. Deicer performance vs. temperature for the eight different brine blends tested.
The results of the laboratory studies are shown in Figure 1. The y-axis represents deicer performance or the rate at which the brine melts ice. The x-axis shows the effects of decreasing temperature. The gray region represents a brine blend made only of NaCl solution mixed with beet juice (Blend 1). The figure indicates that NaCl melts ice very rapidly at temperatures near the freezing point of water. As temperature declines, the rate at which the brine melts ice slows in a nearly linear manner. The blue-shaded regions represent brine solutions which contain CaCl2; as the blend number increases, the quantity of CaCl2 in the brine increases. As temperatures drop, increasing quantities of CaCl2 are added to the brine in order to maintain the desired rate of ice melting. Eight different brine blends were engineered as intended for various temperature ranges. The blends were designed to melt ice at a rate needed to maintain public safety for the temperature range of interest while minimizing cost and chloride application levels.
What was learned
Although deicing material use can be significantly reduced by employing brines, further reductions in materials and cost can be realized by customizing the brine blend to the forecasted weather conditions. The incremental addition of brine CaCl2 greatly reduces the cost and minimizes wasteful use during warmer temperatures when large quantities are unneeded.
Brines can be engineered specifically for pre-wetting granular NaCl, effectively increasing the melting rate at low temperatures. Pre-wetting can be a favorable cost-reducing alternative to purchasing pre-mixed or coated granular material. Pre-wetting ensures the ratio of NaCl to CaCl2 or MgCl2 is uniform, which is not guaranteed when granular materials are mixed using in-house methods. In addition, pre-wetting prevents bounce, scatter and blowing of the granular material during the application process.
The results of the effort to reduce deicing chemical use have been successful and are anticipated to continue improving in the 2015-2016 winter season. Trucks that spread granular material were recalibrated to apply a maximum of 300 lb per lane-mile of NaCl pre-wetted with brine at a rate of 20 gal per ton compared to the previous application rates of up to 1,000 lb per lane-mile. This measure greatly reduced chloride loadings and the impact of deicing chemicals on the environment. The city’s future goal is to further reduce the application rate of granular material and increase the quantity of brine used for pre-wetting. Lincoln’s average in-house production costs for brine using CaCl2 and NaCl are 66% cheaper than purchasing the same amount of proprietary MgCl2 brine. This savings represents a significant potential cost-reduction in anti-icing and deicing chemicals. WM